Embedding an electromagnetic (EM) sensing at the tip of a fetoscope is appealing as it would allow to record the position and orientation of the tip of the fetoscope in real time. This tracking information may be useful for advanced guidance schemes that may help the surgeon during the intervention. However, adding such an EM-sensor should be done with great care as it may lead to additional risks for the patients (mother and fetus), the surgeon or the fetoscope itself. This work proposes the use of a failure mode and effects analysis (FMEA). By means of such an FMEA one can systematically identify the risks and hazards of EM-integration via selective risk assessment. Through this FMEA it is possible to acquire a deeper understanding of the procedure. Together with new guidance schemes that rely on such EM technology, one could ultimately improve patient safety, occupational health safety and ergonomics for the surgeon.
* corresponding author (wipharat.phokee@kuleuven.be, pwipha@kku.ac.th)
Received: 01 March 2020, Accepted: 10 June 2020, Published: 31 December 2020
DOI: 10.5281/zenodo.4383320 | 📁 view PDF file
Background
Twin-to-twin transfusion syndrome (TTTS) is caused by an unbalanced placental flow between identical twins that share their placenta (Lewi, Deprest, and Hecher 2013; Lewi et al. 2012; “Twin-to-Twin Transfusion Syndrome (TTTS)*” 2011). Without intervention, the perinatal mortality rate exceeds 90% due to premature delivery or intra-uterine death, and there may be severe morbidity in survivors (Spruijt et al. 2020; Ahmad et al. 2020; Robyr et al. 2005; M.-V. Senat et al. 2002). Fetoscopic laser ablation (FLA) has become the standard treatment, because of improved outcomes compared with earlier approaches (Marie-Victoire Senat et al. 2004). The embedding of an electromagnetic (EM) sensor in the fetoscope could permit recording the position and orientation of the tip of the fetoscope in real time with a tabletop magnetic field generator placed below the patient. Such information would be beneficial as it would open up possibilities to guide the surgeon intra-operatively and e.g. inform where to laser or how far the placenta is from the fetoscope tip. An advantage of embedding an EM-sensor at the tip of the instrument is that the sensor will stay well inside the range of the magnetic field that is created by the tabletop field generator. However, altering an existing fetoscope and adding EM sensing could lead to additional risks for the patients (mother and fetus), surgeon or the fetoscope. To anticipate and avoid such risks, a selective risk assessment needs to be done. Through an FMEA one could reduce the risks and by introducing further guidance schemes improve patient safety, occupational health safety and ergonomics for the surgeon. A failure mode and effects analysis (FMEA) is a risk assessment tool to identify and analyze risks in healthcare systems. FMEA is a widely accepted method employed both in industry and in the healthcare system(Liu 2019; McElroy et al. 2016). FMEA identifies and analyzes potential failure modes. Typically, a multidisciplinary expert team is involved. The experts should have knowledge and experience with the condition (TTTS), but there should also be experts with technical knowledge on the developed instrumentation. From the assessment by and inputs of the experts, the risks are identified and subsequently ranked. To this end, a risk priority number (RPN) is calculated. The RPN is calculated by multiplying severity (S), occurrence (O) and detectability (D) scores that were assigned by the experts, who indicate how serious the consequences of a failure could be (S), how often such failures could occur (O) and finally how easy it would be to detect and act upon failures (D).
Method
To collect information for FMEA, data was collected in two rounds through a so-called a modified Delphi technique. Five senior engineers were interviewed twice. In round one, potential failure modes were identified via one-to-one interviews. Before the interview the project objectives were communicated to the engineers and the responsibility of the involved clinical staff was explained. In the second round the experts were interviewed with the aim of calculating the different RPN numbers from the different experts.
Result
Embedding an EM-sensor in the fetoscope may lead to a total of 29 additional EM-specific failure modes. The highest number of potential failure modes concerned the failure of the needle assembly, that is used to deliver the EM-sensor and the way the sensor was glued into the needle (17%) followed by failure of the cabling of the EM-sensor (14%), of the needle assembly, the handle (14%), the use of epoxy (14%), failure of the sensor (14%) and finally failure of the fetoscope itself (10%). The fetoscope (30%) had the highest number of potential failure effects (mainly sensor failure due to the presence of metallic components that would disturb the sensor readings), followed by the surgeon (24%) who could experience elevated levels of stress from the potential extension of the intervention and the additional handling, the calibration of the EM system. These were followed by the mother (23%) and the fetus (23%), with potential failures linked to excess strain that could cause cracking or breaking of the cabling. However, after ranking, the highest RPN was for the fetoscope (RPN=48.89;S=4.00), followed by the fetus (RPN=37.33;S=4.67) and mother (RPN=31.5;S=4.33). The highest severity score was 4.67. This related to rupture or cracking of the tube (RPN=37.33;S=4.67), followed by damage to the surgical port during insertion (RPN=21.78;S=4.67) and noise at the EM sensor due to the influence of the needle (RPN=18.67;S=4.67).
Discussion and conclusion
We used a modified Delphi method for a failure mode and effects analysis (FMEA). It identified and investigated potential failure modes and failure effects of embedding an EM-sensor in a fetoscope for laser coagulation of placental vessels. The experts who gave scores also were able to make recommendations for reducing risk factors. Through these recommendations it becomes possible to systematically improve the safety of the design. Experts tended to assign different scores to the different criteria because they had somewhat different backgrounds and preferences. Improvements could be made by finding ways to incorporate expertise to determine the overall RPN. Also, the same RPN number was found for quite different combinations of risk factors (S,O,D). A prioritization depending on individual scores could be adopted to further differentiate among hazards in the future.

Figure 1: The fetoscope, a minimally invasive surgical instrument is inserted through the uterus and fetal membranes under ultrasound to coagulate the placental vessels that are responsible for blood transfusion to the fetus. An EM sensor integrated in a fetoscope could be used to record the position and rotation of the tip of the fetoscope. In order to detect the sensor, a tabletop magnetic field generator is placed below the patient during the procedure.
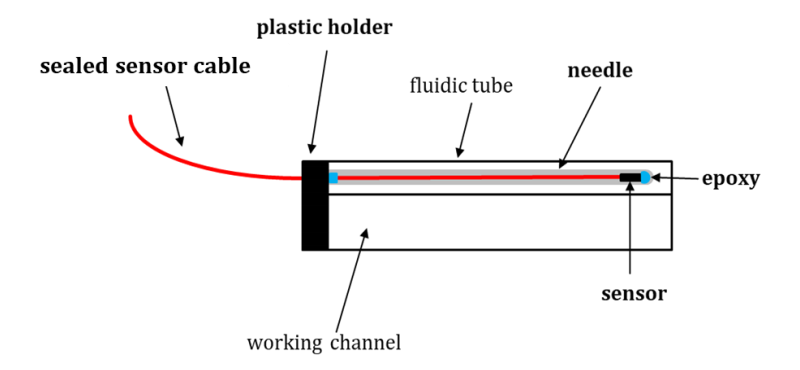
Figure 2: The design of the embedding of an EM sensor in the fetoscope includes a hollow needle, an unsealed 6DOF sensor is inserted inside. The sensor is coated with a water-proof plastic tubing outside the needle. The sensor is sealed inside the needle with epoxy glue. The needle is attached to the fetoscope with a plastic holder. If necessary, the needle is removable from the fluidic tube and separately sterilizable (Kloudová and Mont, 2019).
About the authors
Miss Wipharat is a doctoral student, for this work supervised by Prof. Liliane Pintelon, Department of Mechanical Engineering, Faculty of Engineering Science, KU Leuven and Prof. Emmanuel Vander Poorten, Mechanical Engineering Technology, Group T Leuven Campus, KU Leuven. Mirza Awais Ahmad who designed the EM-integrated needle is supervised by Prof. Jan Deprest, both are affiliated with the cluster Woman and Child, Department of Development and Regeneration of the KU Leuven. Jan Deprest is also a Professor in Obstetrics and Gynaecology at University College London, UK.
Acknowledgements
WP acknowledges the support from the Royal Thai Government Scholarship.
References
- Ahmad, Mirza Awais, Mouloud Ourak, Caspar Gruijthuijsen, Jan Deprest, Tom Vercauteren, and Emmanuel Vander Poorten. 2020. “Deep Learning-Based Monocular Placental Pose Estimation: Towards Collaborative Robotics in Fetoscopy.” International Journal of Computer Assisted Radiology and Surgery 15 (9): 1561–71. https://doi.org/10.1007/s11548-020-02166-3.
- Kloudová, Aneta, and Isabel Mont. n.d. “Final Report H03I7A ‘Design in Medical Technology,’” 27.
- Lewi, Liesbeth, Jan Deprest, and Kurt Hecher. 2013. “The Vascular Anastomoses in Monochorionic Twin Pregnancies and Their Clinical Consequences.” American Journal of Obstetrics and Gynecology 208 (1): 19–30. https://doi.org/10.1016/j.ajog.2012.09.025.
- Lewi, Liesbeth, Roland Devlieger, Luc De Catte, and Jan Deprest. 2012. “Twin-Twin Transfusion Syndrome: The Good News Is; There Is Still Room for Improvement …: Twin-Twin Transfusion Syndrome.” Acta Obstetricia et Gynecologica Scandinavica 91 (10): 1131–33. https://doi.org/10.1111/aogs.12002.
- Liu, Hu-Chen. 2019. Improved FMEA Methods for Proactive Healthcare Risk Analysis. Singapore: Springer Singapore. https://doi.org/10.1007/978-981-13-6366-5.
- McElroy, Lisa M, Rebeca Khorzad, Anna P Nannicelli, Alexandra R Brown, Daniela P Ladner, and Jane L Holl. 2016. “Failure Mode and Effects Analysis: A Comparison of Two Common Risk Prioritisation Methods.” BMJ Quality & Safety 25 (5): 329–36. https://doi.org/10.1136/bmjqs-2015-004130.
- Robyr, R., M. Boulvain, L. Lewi, A. Huber, K. Hecher, J. Deprest, and Y. Ville. 2005. “Cervical Length as a Prognostic Factor for Preterm Delivery in Twin-to-Twin Transfusion Syndrome Treated by Fetoscopic Laser Coagulation of Chorionic Plate Anastomoses: Preterm Delivery in TTTS.” Ultrasound in Obstetrics and Gynecology 25 (1): 37–41. https://doi.org/10.1002/uog.1798.
- Senat, Marie-Victoire, Jan Deprest, Michel Boulvain, Alain Paupe, Norbert Winer, and Yves Ville. 2004. “Endoscopic Laser Surgery versus Serial Amnioreduction for Severe Twin-to-Twin Transfusion Syndrome.” New England Journal of Medicine 351 (2): 136–44. https://doi.org/10.1056/NEJMoa032597.
- Senat, M.-V., J.-P. Bernard, S. Loizeau, and Y. Ville. 2002. “Management of Single Fetal Death in Twin-to-Twin Transfusion Syndrome: A Role for Fetal Blood Sampling: Single Fetal Death in TTTS.” Ultrasound in Obstetrics and Gynecology 20 (4): 360–63. https://doi.org/10.1046/j.1469-0705.2002.00815.x.
- Spruijt, Marjolijn S., Enrico Lopriore, Sylke J. Steggerda, Femke Slaghekke, and Jeanine M.M. Van Klink. 2020. “Twin-Twin Transfusion Syndrome in the Era of Fetoscopic Laser Surgery: Antenatal Management, Neonatal Outcome and Beyond.” Expert Review of Hematology 13 (3): 259–67. https://doi.org/10.1080/17474086.2020.1720643. “Twin-to-Twin Transfusion Syndrome (TTTS)*.” 2011. Journal of Perinatal Medicine 39 (2). https://doi.org/10.1515/jpm.2010.147.
License
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0) License, which permits to copy and redistribute the material in any medium or format. You are also allowed to remix, transform, and build upon the material under the following terms: 1) You must give appropriate credit, provide a link to the license, and indicate if changes were made. 2) You may not use the material for commercial purposes. 3) If you remix, transform or build upon the material, you must distribute your contributions under the same license as the original. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc-sa/4.0/