Nitrite reduction by Xanthine Oxidoreductase modulates platelet reactivity and in part mediates platelet inhibition by inorganic nitrate in mice
Xanthine oxidoreductase (XOR) is a potential missing link in platelet hyperactivity and cardiovascular diseases. Bioconversion of NO2- to NO by XOR modulates platelet reactivity as we observed rises in plasma NO2- levels after Xdh allele deletion in mice. This higher plasma NO2- in Xdh heterozygous mice was associated with greater platelet reactivity demonstrated in a tail bleeding, thrombus formation, and platelet aggregation study. There is also a potential of nitrate on platelet inhibition in wild-type mice, which developed smaller thrombus formation.
* corresponding author (a.ahluwalia@qmul.ac.uk)
Received: 24 May 2020, Accepted: 02 June 2020, Published: 31 December 2020
DOI: 10.5281/zenodo.4383347 | 📁 view PDF file
Background
Inorganic nitrate (NO3-) is a stable form of nitric oxide (NO) found in green leafy vegetables and beetroots. NO3- has been proposed as a potential therapy to improve cardiac function and decrease platelet-hyperactivity (Velmurugan et al. 2013, Velmurugan et al. 2016). These effects are based upon the capacity for NO3- to be converted to nitrite (NO2-) and further NO in the body. Whilst the conversion of NO3- to NO2- is governed by the host commensal bacteria, the conversion of NO2- to NO is facilitated by mammalian NO2- reductases (Lundberg, Weitzberg, and Gladwin 2008) (Figure 1A). One particular prominent NO2- reductase is xanthine oxidoreductase (XOR), however, whether this pathway plays a role in the effects of NO2- upon platelet activity is unknown. Thus, we assessed the role of XOR in mediating NO2- induced platelet inhibition using Xdh genetically deleted mice to assess plasma NO2- concentrations and platelet reactivity. In addition, the impact of Xdh deletion upon two-week NO3- supplementation in drinking water (15mM) on platelet functions was also determined.
Method
Wild type (WT), Xdh heterozygous (Het), and Xdh knock out (KO) mice were used to assess plasma NO2- by chemiluminescence and platelet reactivity. In vivo platelet function was assessed using tail bleeding (N=15) and a ferric chloride model of thrombosis using intravital microscopy (N=7), while platelet aggregation responses to collagen or protease-activated receptor-4 activating peptide (PAR4-AP) was measured using impedance aggregometry ex vivo (N=15). To explore the possibility that deletion of the gene impacted upon the effects of dietary NO3-, mice were treated with KNO3 in drinking water (15 mM) or equimolar salt (15mM KCl) as a control for 2 weeks prior to assessing plasma NO2- concentrations, and thrombus formation (N=7). Because Xdh KO mice did not survive beyond 4 weeks, we were unable to use Xdh KO mice in all experiments.
Result
After Xdh allele deletion, rises in plasma NO2- levels were observed suggesting lack of conversion of NO2- to NO (Figure 1B). Xdh Het and KO mice expressed shorter tail bleeding times compared to WT mice. Xdh Het mice developed greater thrombosis responses to ferric chloride and increased platelet aggregation induced by collagen compared to WT mice (Figure 1C). Moreover, NO3- administration had the potential to increase NO2- levels in both WT and Het mice. However, this associates with decreased thrombosis responses in WT mice only.
Discussion and conclusion
Here we show for the first time that with each allele deletion there is a rise in nitrite levels in plasma. We speculate that this rise in nitrite levels may relate to a loss of nitrite processing from XOR to NO. Longer tail bleeding time, smaller thrombus formation and lower platelet aggregation observed in WT mice suggesting a role for XOR in mediating NO production and platelet activity. In addition, there is a potential of nitrate on platelet inhibition mediated by XOR.
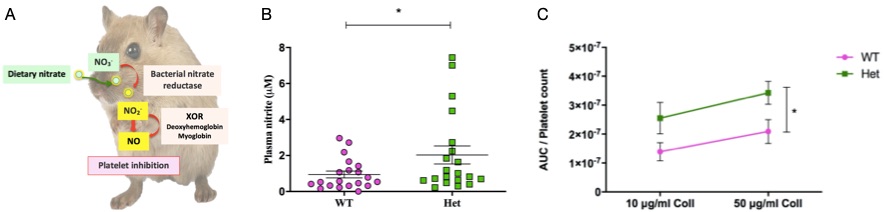
Figure 1: Role of XOR, plasma nitrite, and platelet aggregation in the Xdh+/- colony. XOR is responsible for bioconversion of NO2- to NO, which potentially modulates platelet activation (A). In this study, plasma collected from Xdh WT and Het mice was measured for nitrite by chemiluminescence (B). Platelet aggregation by collagen were recorded by impedance aggregometry and normalized to platelet count (C). Data are mean ± SEM. For plasma nitrite n=20 and platelet aggregation n=15. Statistical significance was determined using an unpaired T-test (A), and Two-way ANOVA followed by Bonferroni’s multiple posttests (B) represented by *P < 0.05.
About the authors
Ms Parakaw is a PhD student in Prof Ahluwalia laboratory at the William Harvey Research Institute, Barts and London Medical School, Queen Mary University of London, London, UK. This research is conducted under the supervision of Prof Ahluwalia, professor of pharmacology and Mr Khambata, a lecturer in vascular pharmacology at the William Harvey Research Institute. Prof Ahluwalia is the major contributor to the idea and project design and Ms Parakaw contributes to all laboratory experiments.
Acknowledgements
This project has received funding of the EU Horizon 2020 research and innovation program under Marie Sklodowska Curie grant agreement NO. 675111
References
Lundberg, J. O., E. Weitzberg, and M. T. Gladwin. 2008. “The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics.” Nat Rev Drug Discov no. 7 (2):156-67. Velmurugan, S., J. M. Gan, K. S. Rathod, R. S. Khambata, S. M. Ghosh, A. Hartley, S. Van Eijl, V. Sagi-Kiss, T. A. Chowdhury, M. Curtis, G. G. Kuhnle, W. G. Wade, and A. Ahluwalia. 2016. “Dietary nitrate improves vascular function in patients with hypercholesterolemia: a randomized, double-blind, placebo-controlled study.” Am J Clin Nutr no. 103 (1):25-38. Velmurugan, S., V. Kapil, S. M. Ghosh, S. Davies, A. McKnight, Z. Aboud, R. S. Khambata, A. J. Webb, A. Poole, and A. Ahluwalia. 2013. “Antiplatelet effects of dietary nitrate in healthy volunteers: involvement of cGMP and influence of sex.” Free Radic Biol Med no. 65:1521-32.
License
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0) License, which permits to copy and redistribute the material in any medium or format. You are also allowed to remix, transform, and build upon the material under the following terms: 1) You must give appropriate credit, provide a link to the license, and indicate if changes were made. 2) You may not use the material for commercial purposes. 3) If you remix, transform or build upon the material, you must distribute your contributions under the same license as the original. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc-sa/4.0/